Last update images today 510 K Predicate Device


 predicate device.png)




+social.jpg)











+device+to+a+similar+legally+marketed+device..jpg)


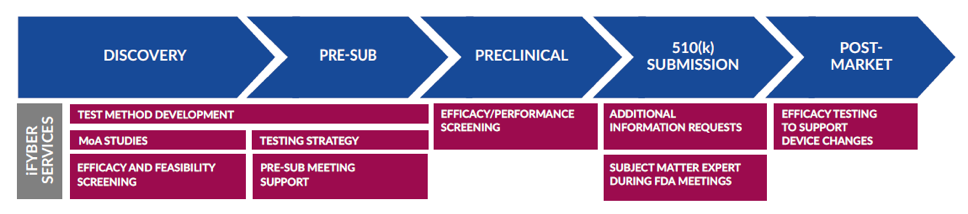







https secureservercdn net 160 153 137 170 hmm cf5 myftpupload com wp content uploads 2020 02 FDA 510k System And 510 K Premarket Notification Devices jpg - The FDA 510 K Premarket Notification Of Devices BRG FDA 510k System And 510 K Premarket Notification Devices https www eclevarmedtech com wp content uploads 2023 01 needing unique solutions 1 png - The 510 K Approval Route What Do You Need To Know Needing Unique Solutions. 1
https static complianceonline com images articles 706580 jpg - The 510 K Application 5 Best Practices Medical Device Companies Must 706580 https simbex com wp content smush webp 2024 06 the role of predicate devices in 510k submissions simbex jpg webp - The Role Of Predicate Devices In 510 K Submissions Simbex The Role Of Predicate Devices In 510k Submissions Simbex .webphttps podcast easymedicaldevice com wp content uploads 2023 11 LinkedIN episode259 2x3 Low jpg - How To Find Your Predicate Device For Your 510K Submission LinkedIN Episode259 2x3 Low
https assets global website files com 5ab16d21eba35cdb2416f449 61dc6a30f3f3df7e668b062e 510k program article heading december 2021 png - The 510 K Program All About This Submission Type 61dc6a30f3f3df7e668b062e 510k Program Article Heading December 2021 https clariscience com wp content uploads 2024 02 011 Q FDA 510k jpg - Come Si Seleziona Un Predicate Device Clariscience 011 Q FDA 510k
https operonstrategist com wp content uploads 2023 04 6 Tips to Use a Predicate Device Effortlessly 01 1536x803 jpg - 6 Tips To Locate And Use A Predicate Device Effortlessly For 510k 6 Tips To Use A Predicate Device Effortlessly 01 1536x803