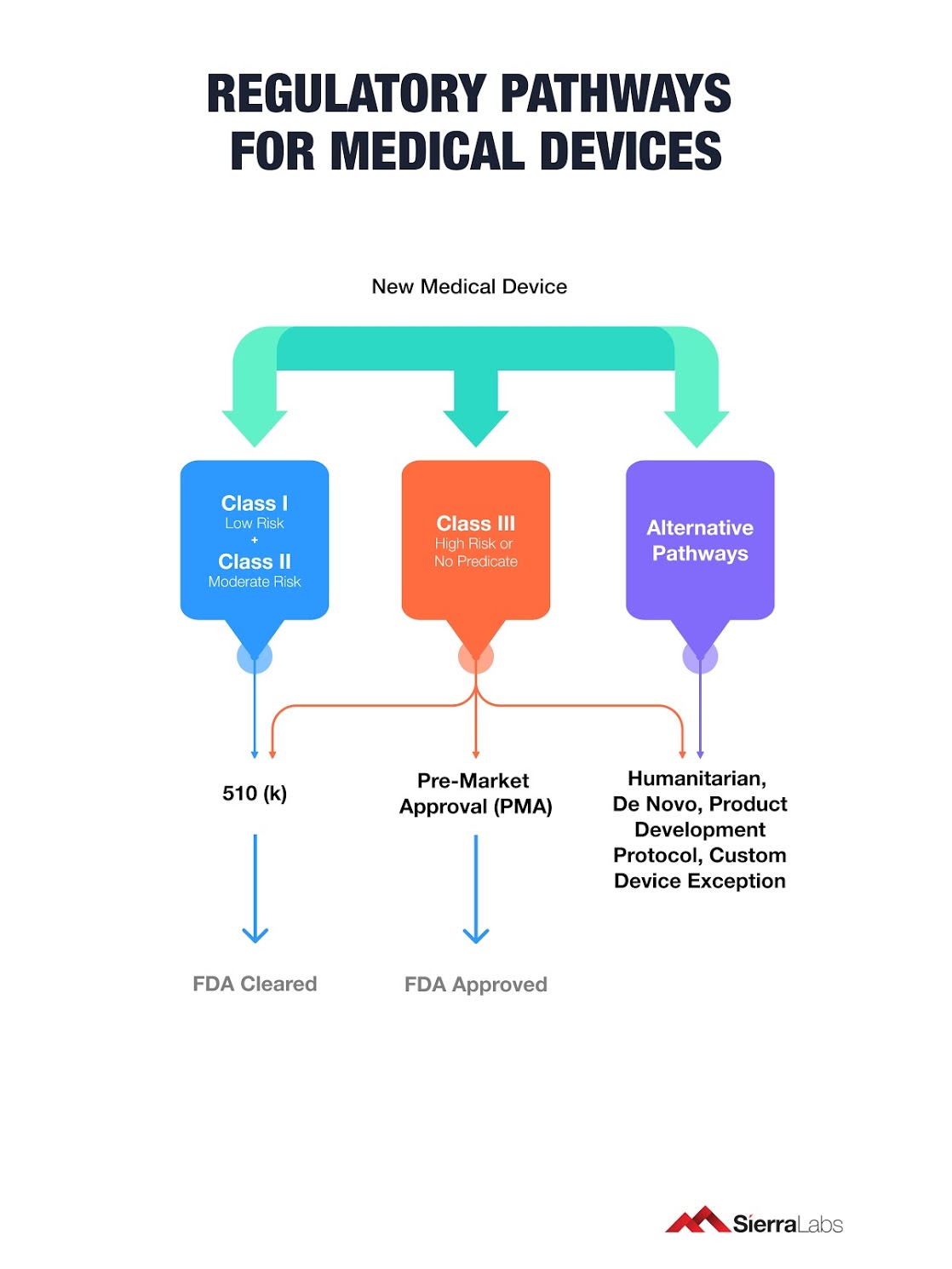
Last update images today 510 K Regulatory Pathway



.jpg)













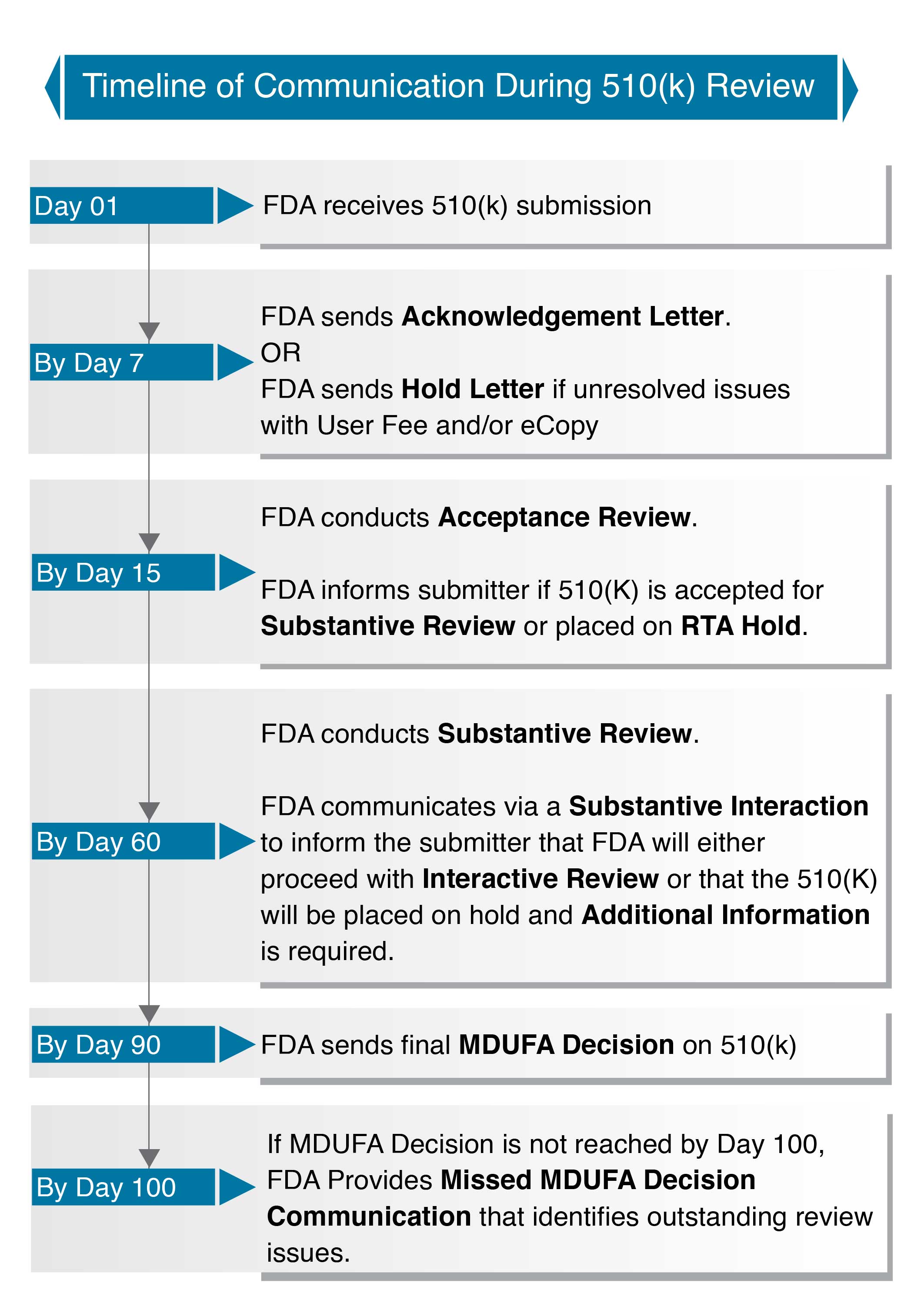










https about citiprogram org wp content uploads 2022 09 510k subs jpg - New FDA Guidance For Medical Device 510 K Submissions 510k Subs https www ncbi nlm nih gov books NBK209655 bin p2001d9c5g78001 jpg - 510 K Premarket Notification Analysis Of FDA Recall Data Public P2001d9c5g78001
https www massdevice com wp content uploads 2019 09 medical device pathway png - Niektor Zn Me Podrobnosti O Lie Be Liekov Solvay Park Medical Device Pathway https www regdesk co wp content uploads 2022 10 istockphoto 1169753157 612x612 1 jpeg - FDA Guidance On Abbreviated 510 K Program Overview RegDesk Istockphoto 1169753157 612x612 1 https www eclevarmedtech com wp content uploads 2023 01 needing unique solutions 1 300x300 png - The 510 K Approval Route What Do You Need To Know Needing Unique Solutions. 1 300x300
https images prismic io medtruth 6242443f c52f 446b 92ab c30df7cee84a 510 k jpg - The 510 K Approval Process A Guide MedTruth Prescription Drug 6242443f C52f 446b 92ab C30df7cee84a 510(k) https www amjmed com cms asset f225beaf 682b 440d a35a 635d3376422d gr1 jpg - The Food And Drug Administration S FDA S 510 K Process A Systematic Gr1
https operonstrategist com egypt wp content uploads 2022 11 FDA 510k Consultant 01 1024x536 jpg - FDA 510k Clearance Submission And Premarket Approval FDA 510k FDA 510k Consultant 01 1024x536