Last update images today 510k Premarket Notification








.jpg)


















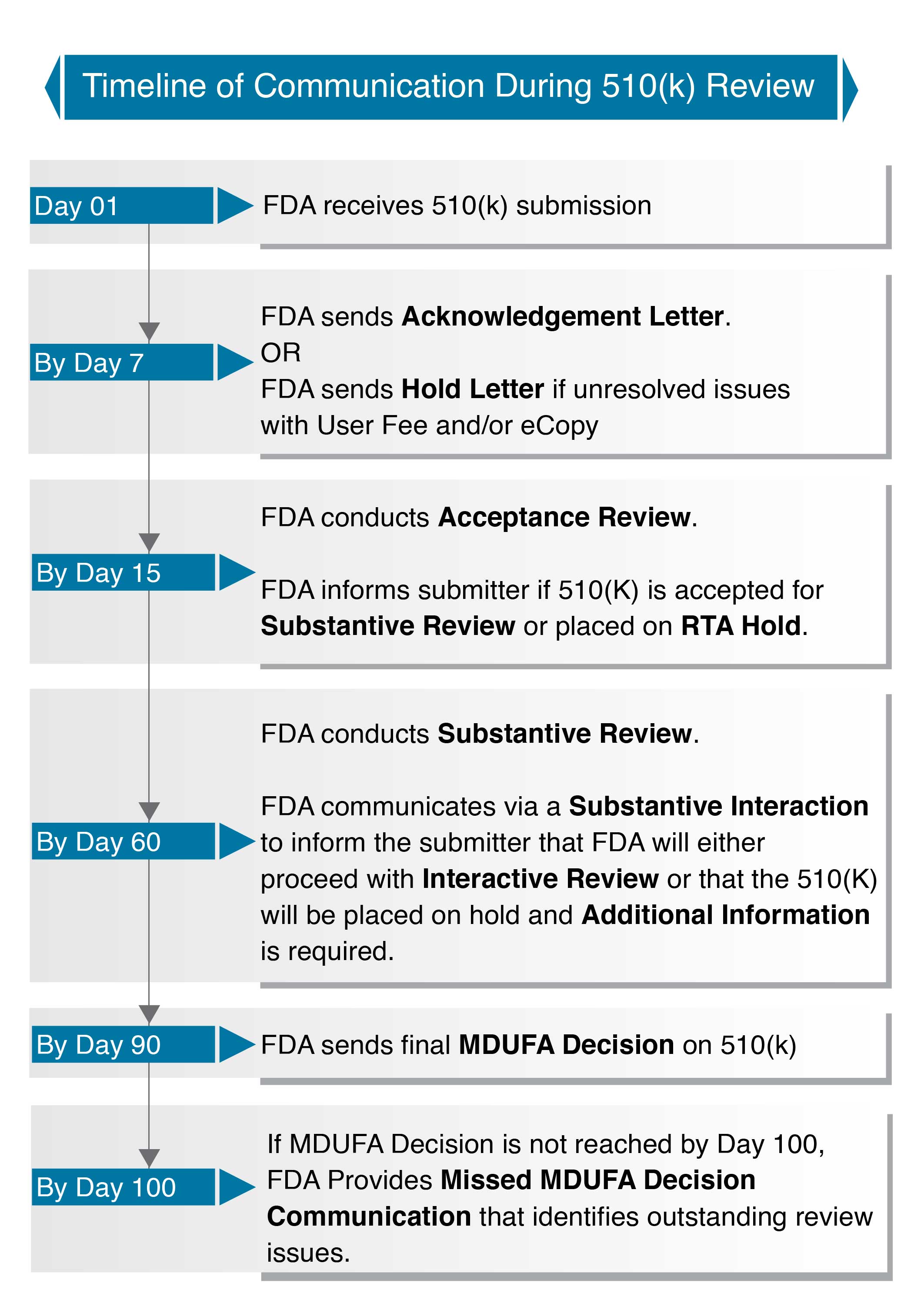



https www thefdagroup com hubfs fda WP Ad 501kSubmissions 01 jpg keepProtocol - An Expert Guide To FDA 510 K Submissions Fda WP Ad 501kSubmissions 01 #keepProtocolhttps assets global website files com 6141b0efd8e313f1168ba8cc 61a56552e33fbc0c49693790 fda 510k thumb jpg - The 510 K Application If Content Is King Then Communication Is Queen 61a56552e33fbc0c49693790 Fda 510k Thumb
https assets website files com 5ab16d21eba35cdb2416f449 63d14e898ac87b725881d42e 2eMWyMDjPlgF5lpBNfyWvHj4Guk7tbkzWjaNXoCMKkSD7TnshoyM2t9ZrwqqEIiWDRIQs4fq1oMEZZVFCd8EYOCIZnT63x4imrJeTwdNyaIB2bBerYL8Hy8MX 7VHzomkorCoXSBj7oxQRs 8Plt7 rM6tG5RXlkn5OiI33ZMCLo7BFsZsQm2AS lh2ERnHO2HnvJw0y1g png - 510 K Or PMA Should Your Medical Device Receive FDA Clearance Or FDA 63d14e898ac87b725881d42e 2eMWyMDjPlgF5lpBNfyWvHj4Guk7tbkzWjaNXoCMKkSD7TnshoyM2t9ZrwqqEIiWDRIQs4fq1oMEZZVFCd8EYOCIZnT63x4imrJeTwdNyaIB2bBerYL8Hy8MX 7VHzomkorCoXSBj7oxQRs 8Plt7 RM6tG5RXlkn5OiI33ZMCLo7BFsZsQm2AS Lh2ERnHO2HnvJw0y1g https images prismic io medtruth 6242443f c52f 446b 92ab c30df7cee84a 510 k jpg - The 510 K Approval Process A Guide MedTruth Prescription Drug 6242443f C52f 446b 92ab C30df7cee84a 510(k) https medicaldevicelicense com wp content uploads 2023 09 fda 510k submission consulting services in india md jpg - Best FDA 510k Submission Consulting Services In Baddi HP Fda 510k Submission Consulting Services In India Md
https secureservercdn net 160 153 137 170 hmm cf5 myftpupload com wp content uploads 2020 02 FDA 510k System And 510 K Premarket Notification Devices jpg - The FDA 510 K Premarket Notification Of Devices BRG FDA 510k System And 510 K Premarket Notification Devices https miro medium com v2 resize fit 832 0 srsdAsXiaPDQ1iV6 png - FDA 510 K Clearance Premarket Approval By Operon Strategist Medium 0*srsdAsXiaPDQ1iV6
https qualitysmartsolutions com wp content uploads 2023 01 QSS Images 2023 01 23T143432 362 png - 510k Process Regulatory Hurdles 510K Premarket Notification QSS Images 2023 01 23T143432.362